Abstract
The bloodstream of mice and humans contain consistent proportions of red blood cells (RBC) which are bound to platelets (1-2% and 0.1-0.3%, respectively). This study identifies senescent RBC as the main constituent of platelet-RBC complexes (Plt-RBC) and their importance in RBC clearance. RBC have a defined circulation lifespan and upon reaching senescence are eliminated by phagocytes, thereby ensuring homeostasis and preventing accumulation of dead cells and toxic hemolytic byproducts. Senescent RBC are characterized by cellular and molecular changes, including reduced deformability, externalization of membrane phosphatidylserine (PS), and altered expression of membrane proteins such Fas ligand (CD95; upregulated) and integrin associated protein (CD47; downregulated), which act as pro-phagocytic signals. Similar changes to deformability and membrane composition occur in RBC compromised by sickle cell disease or Plasmodium infection. These pathologic cells also display a 10x higher platelet-binding capacity which is related to disease pathogenesis. Thus, we investigated how platelets may interact with senescent RBC and the purpose of Plt-RBC.
Platelet binding to senescent RBC was studied using mice pulse-labelled with biotin-NHS, which enabled tracking of RBC age and clearance over time. The biotin+ RBC remaining for longer times represented older cells; the oldest detected were aged ≥58 days consistent with the reported maximal RBC lifespan in mice. During the first 45 days of observation Plt-RBC were similar in biotin+ versus newly replenished biotin- RBC (1.6-2.2%), but were significantly higher in biotin+ cells detected at 52 and 58 days (average ± SD: 6.7 ± 5.1% and 15 ± 8.1%, respectively; p < 0.001). These observations were replicated when biotin-NHS was replaced with CFSE. The aged RBC and Plt-RBC also contained significantly increased PS and CD95 compared to the replenished and non-complexed cells, although CD47 levels were not different. Flow cytometry imaging revealed Plt-RBC were composed of single RBC bound by 1-2 platelets. Collectively this indicated that platelets preferentially bind senescent RBC.
To investigate if platelet binding affected senescent RBC clearance, platelet-depleting antibodies (anti-GPIbα or anti-GPIIb/IIIa) were given to temporarily reduce Plt-RBC when biotin+ Plt-RBC levels first began to diverge (Day 46-49 in multiple experiments). The treatment caused a maximal 10-fold reduction in Plt-RBC levels sustained for 2-3 days, and a marked retention of biotin+ RBC which coincided with Plt-RBC reductions. Consistent with these results, the clearance of transfused RBC in platelet-depleted mice was also significantly inhibited compared to control mice. Erythrophagocytes in the spleen, but not the liver, from the platelet-depleted mice also contained fewer transfused cells, and erythrophagocytes containing platelets were observed using flow cytometry and microscopy. Thus, platelets are important for the clearance and/or phagocytosis of both senescent and transfused RBC. We also studied the Plt-RBC clearance mechanism by transfusing ex vivo generated and labelled Plt-RBC and RBC. The Plt-RBC were cleared at least 5x faster than RBC (p=0.0014) suggesting that once formed, their lifespan in the circulation is transient. Similar transfusions in mice depleted of phagocytes (using clodronate liposomes) impeded Plt-RBC clearance, indicating a phagocyte-dependent pathway for their removal. An additional mechanistic insight, Plt-RBC displayed an increased retention in a microsphiltration assay system, indicating platelet binding may hinder the passage of cells through the microcirculation.
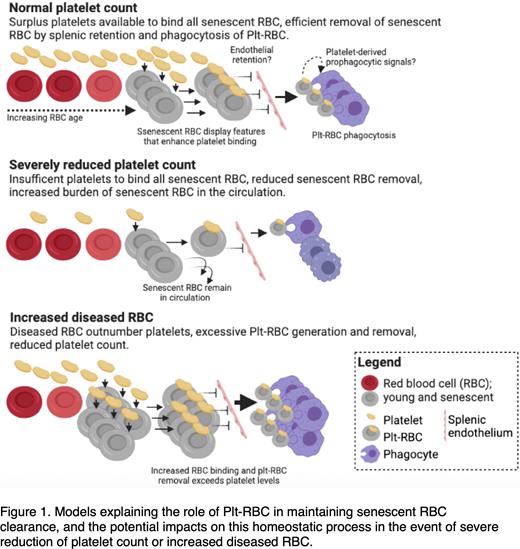
Our studies have thus revealed an unexpected contribution by platelets in senescent RBC clearance. In our proposed model (Figure 1) senescent RBC have an enhanced ability to bind platelets, possibly due to upregulated PS and CD95, which accelerates their removal via splenic retention and phagocytosis. Platelets may also present additional pro-phagocytic signals to stimulate erythrophagocytosis. A normal platelet count provides surplus platelets to maintain RBC clearance, but in conditions of acute platelet loss, reduction of plt-RBC may cause accumulation of senescent RBC to pathological levels. Conversely, conditions where more RBC bind platelets such as in malarial infection will increase plt-RBC and result in thrombocytopenia.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.